Is light a wave or a particle or is the reality even weird?
Photoelectric Effect explained...
Greetings, fellow scientist
It's an old age question and a matter of debate in ancient times of what light exactly is. Of the four incredible Annus Mirabilis papers by Einstein, the first one tried to solve this issue by advancing on some of the previously developed theories.
Historical Background
In 1672 Robert Hooke and Isaac Newton were at each other's throats regarding the true nature of light. Newton argued that the geometric nature of reflection and refraction of light could only be explained if light were made of particles. He further said that waves do not travel in a straight line. Whereas Robert Hooke was an advocate of the wave theory of light which held that light was made up of white-coloured wavelengths.
In 1801 through his famous double-slit experiment, Thomas Young demonstrated the wave nature of light and thus proved Newton wrong.

Then in 1865 Maxwell's equations of light proved light as an electromagnetic wave.
Hence, everyone thought that the age-old debate of the nature of light came to an end. But not so soon.
In 1887 Henrich Hertz found that electric charge was discharged when cathode was illuminated by ultraviolet light.
Further, in 1898 J.J Thompson demonstrated that electrons are ejected from the metal surface when ultraviolet light was thrown on it. Then present theories failed to explain this phenomenon and it was unclear what the explanation could be.
Physics of Photoelectric Effect
It was not until 1905 that this puzzle was solved by Albert Einstein in his paper “On a Heuristic Viewpoint Concerning The Production And Transformation Of Light” which was received by the journal on 18th March 1905 and published on 9th June 1905. He named this phenomenon the photoelectric effect.
Photoelectric effect is the emission of electrons when electromagnetic radiation, such as light, strikes a material.

Now photoelectric effect can’t be explained by the wave nature of light but by particle nature. This effect occurs as a result of light energy being carried in discrete quantized packets known as photons. The energy held by each photon is directly proportional to the frequency of light. The light of different frequencies carry photons of different energies. More is the frequency of light, more will be the energy carried by the photons.

Since light is composed of photons, when they fall on the metal surface each photon transfers its entire energy to each electron. If the energy of the photon is equal to or more than that of work function (minimum quantity required to remove an electron from the surface of a given solid) then the electron is ejected and the rest of the energy is converted to the kinetic energy of the electron.
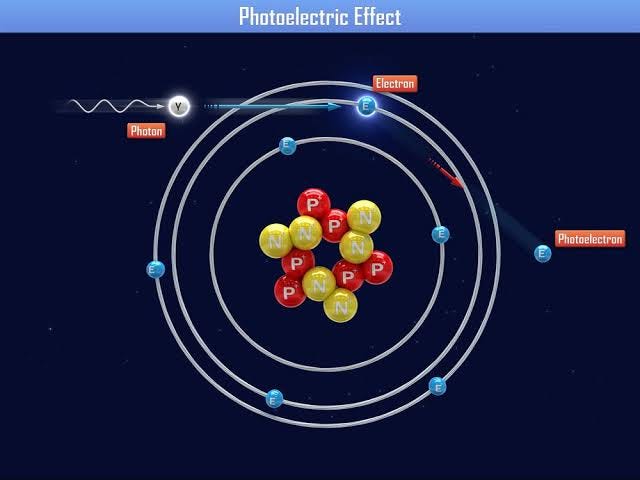
An electron’s kinetic energy only depends on the frequency of light used and not on its intensity as each photon transfers its energy to each electron.
Now by increasing the intensity of light, the number of photons also increases hence more electrons can be ejected. Therefore by increasing the intensity of light the current flow increases but do not affect the speed of electrons.
Applications

This phenomenon helps to generate electricity using photoelectric cells which convert sunlight into electricity. It is also useful to develop night vision devices using semiconductors. This effect is also responsible for an apparent dusty atmosphere of the moon caused by the electrostatic levitation of the positively charged dust particles from the surface.
The photoelectric effect proved the wave-particle duality of light according to which light consists of tiny particles which have wave-like properties. It opened the realm of Quantum Mechanics.
In 1921, Einstein was awarded the Nobel Prize in Physics for this notable discovery, although there were still three more papers to go.



loved it
Good explanation 👍👍